Is Galvanic Good . learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. learn the basics of redox reactions and how to apply them to electrochemical cells. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. describe the function of a galvanic cell and its components; galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. Use cell notation to symbolize the composition and. galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. A galvanic cell, also called a voltaic cell, produces electricity from.
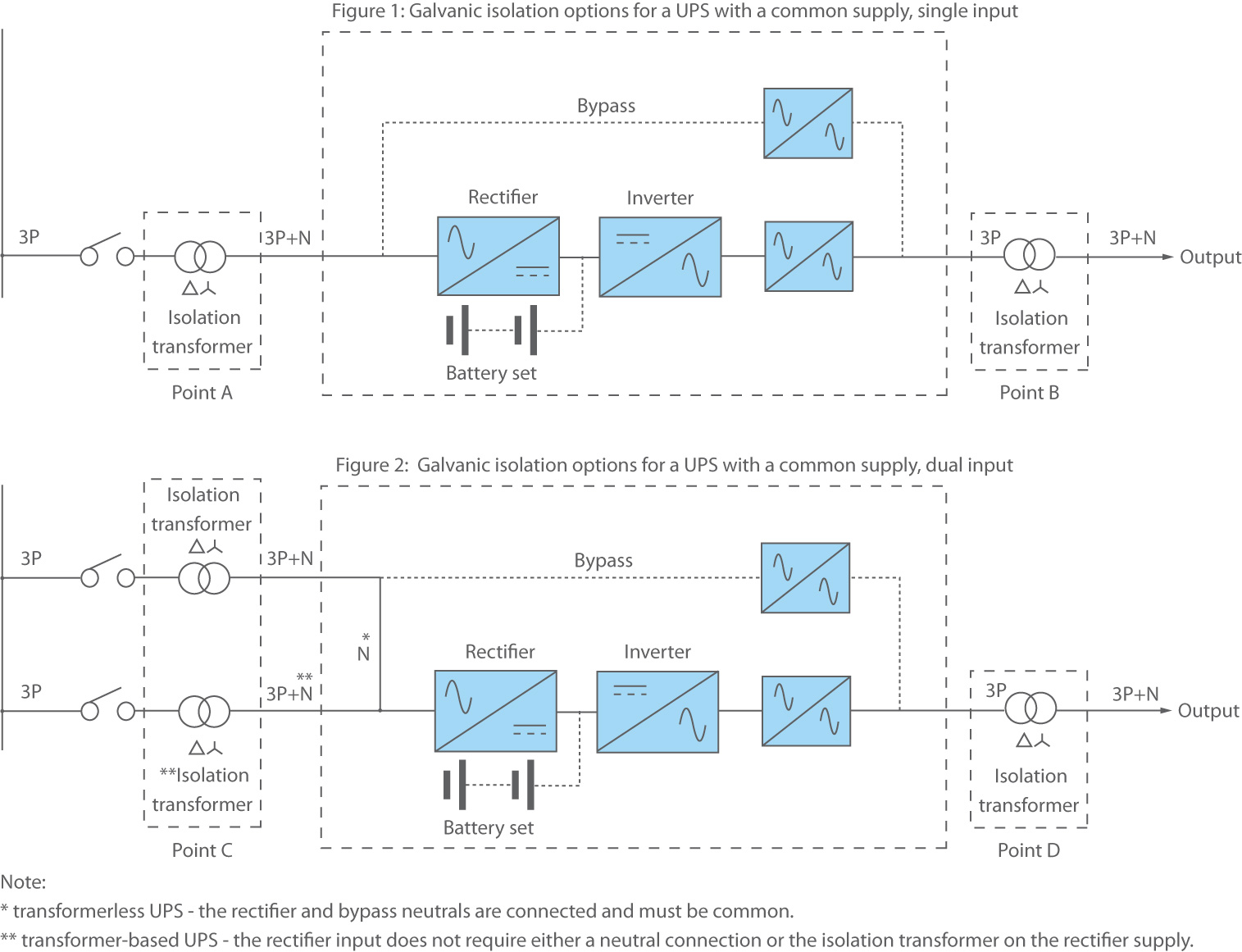
from www.riello-ups.co.uk
galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. Use cell notation to symbolize the composition and. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. describe the function of a galvanic cell and its components; A galvanic cell, also called a voltaic cell, produces electricity from. galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. learn the basics of redox reactions and how to apply them to electrochemical cells.
What Is Galvanic Isolation?
Is Galvanic Good galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. A galvanic cell, also called a voltaic cell, produces electricity from. galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. Use cell notation to symbolize the composition and. describe the function of a galvanic cell and its components; learn the basics of redox reactions and how to apply them to electrochemical cells.
From gachalifeonline.heroinewarrior.com
10 Amazing Beauty Benefits Of Galvanic Facial You Need To Know Is Galvanic Good learn the basics of redox reactions and how to apply them to electrochemical cells. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. A galvanic cell, also called a voltaic cell, produces electricity from. Use cell notation to symbolize the composition and. galvanic cells are electrochemical cells that produce electricity by using different metals. Is Galvanic Good.
From www.engineeringclicks.com
The Galvanic Series the essential guide EngineeringClicks Is Galvanic Good A galvanic cell, also called a voltaic cell, produces electricity from. Use cell notation to symbolize the composition and. galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. galvanic cells are electrochemical cells that produce electricity by. Is Galvanic Good.
From www.corrosionpedia.com
How Galvanic Corrosion Can Be Used for Good Is Galvanic Good learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. A galvanic cell, also called a voltaic cell, produces electricity from. galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. learn the basics of redox reactions and how to apply them to electrochemical. Is Galvanic Good.
From www.vrogue.co
What Is Galvanic Corrosion Galvanic Corrosion Chart vrogue.co Is Galvanic Good galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. Use cell notation to symbolize the composition and. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. A galvanic cell, also. Is Galvanic Good.
From www.purechemistry.org
Galvanic cell Is Galvanic Good Use cell notation to symbolize the composition and. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. learn the basics of redox reactions and how to apply them to electrochemical cells. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. A galvanic cell, also called a voltaic. Is Galvanic Good.
From www.chegg.com
Solved (a) Figure 1 below represents the galvanic series. Is Galvanic Good Use cell notation to symbolize the composition and. galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. A galvanic cell, also called a. Is Galvanic Good.
From www.yourbeautygadgets.com
Galvanic In Cosmetology And Its BenefitsYour Beauty Gadgets Is Galvanic Good Understand the role of ions, current, charge, and salt bridge in electrochemical cells. Use cell notation to symbolize the composition and. A galvanic cell, also called a voltaic cell, produces electricity from. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. galvanic corrosion is an electrochemical process in which one metal. Is Galvanic Good.
From www.collegesearch.in
Galvanic Cells Definitions, Examples, How They are Created, Principles Is Galvanic Good Use cell notation to symbolize the composition and. A galvanic cell, also called a voltaic cell, produces electricity from. describe the function of a galvanic cell and its components; galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. learn how galvanic cells operate by measuring the potential difference between two electrodes in. Is Galvanic Good.
From thosegraces.com
Galvanic vs Microcurrent What Are Their Key Differences? Is Galvanic Good galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. describe the function of a galvanic cell and its components; Use cell notation to symbolize the composition and. learn how galvanic cells operate by measuring the potential. Is Galvanic Good.
From engineerexcel.com
Galvanic Corrosion [with Chart] EngineerExcel Is Galvanic Good Use cell notation to symbolize the composition and. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. describe the function of a galvanic cell and its components; galvanic cells are electrochemical cells that produce electricity by using. Is Galvanic Good.
From melscience.com
Daniell galvanic cell MEL Chemistry Is Galvanic Good galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. describe the function of a galvanic cell and its components; galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. A galvanic cell, also called a voltaic cell, produces electricity from. learn how galvanic cells. Is Galvanic Good.
From www.icorr.org
Galvanic Corrosion the importance of designingout corrosion hotspot Is Galvanic Good Use cell notation to symbolize the composition and. A galvanic cell, also called a voltaic cell, produces electricity from. learn the basics of redox reactions and how to apply them to electrochemical cells. describe the function of a galvanic cell and its components; galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes.. Is Galvanic Good.
From thegoodchemistguide.blogspot.com
The Good Chemist's Guide Copper Zinc Galvanic Cell Is Galvanic Good galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. Use cell notation to symbolize the composition and. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. learn how galvanic cells operate. Is Galvanic Good.
From www.intermarineboats.com
Galvanic Corrosion InterMarine Boats Is Galvanic Good galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. A galvanic cell, also called a voltaic cell, produces electricity from. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. galvanic corrosion is. Is Galvanic Good.
From www.riello-ups.co.uk
What Is Galvanic Isolation? Is Galvanic Good learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. describe the function of a galvanic cell and its components; Understand the role of ions, current, charge, and salt bridge in electrochemical cells. A galvanic cell, also called a voltaic cell, produces electricity from. learn the basics of redox reactions and. Is Galvanic Good.
From www.slideserve.com
PPT Galvanic Current PowerPoint Presentation, free download ID6670597 Is Galvanic Good Use cell notation to symbolize the composition and. galvanic cells are electrochemical cells that produce electricity by using different metals and electrolytes. learn the basics of redox reactions and how to apply them to electrochemical cells. Understand the role of ions, current, charge, and salt bridge in electrochemical cells. galvanic corrosion is an electrochemical process in which. Is Galvanic Good.
From www.youtube.com
what is a Galvanic cell ? YouTube Is Galvanic Good describe the function of a galvanic cell and its components; learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions. galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical. A galvanic cell, also called a voltaic cell, produces electricity from. galvanic cells. Is Galvanic Good.
From www.jlconline.com
Separating Galvanic Metals JLC Online Is Galvanic Good Understand the role of ions, current, charge, and salt bridge in electrochemical cells. Use cell notation to symbolize the composition and. describe the function of a galvanic cell and its components; A galvanic cell, also called a voltaic cell, produces electricity from. learn how galvanic cells operate by measuring the potential difference between two electrodes in different solutions.. Is Galvanic Good.